Today we delve into soil chemistry and biology. If you dreaded chemistry in school, fear not - there will be no chemical equations or molecular formulae or anything like that, just a few topics I think are essential for understanding your soil. If you love chemistry, check out the links at the end to learn more. The biology section will take a brief glimpse at all the life, both visible, and microscopic, in your soil, and how most of it interacts with your plants in a beneficial way. If you haven't read the previous two posts of this series, I recommend you check out our Intro and Physical Qualities of Soil.
Chemistry: pH and CEC
Soil pH is a measure of the amount of positively charged hydrogen ions in a mixture, and in practical application it tells us how acidic something is. Values range from 0-14, with 0 being extremely acidic and 14 being extremely basic or alkaline. Most plants like pH ranges from about 5.5-7, or slightly acidic to neutral. Central Indiana has soils that developed from limestone, so we usually see soil pH that is closer to neutral or even alkaline. If your property has had recent construction, you could see a temporary increase in alkalinity. Soil pH matters because it affects nutrient availability for plants, or the plant's ability to absorb the nutrients present in the soil. Most of the elements a plant needs are taken into the roots as positively or negatively charged particles called ions. These nutrient ions interact with the soil differently depending on the pH. At a neutral or slightly acidic pH, the greatest amount of plant nutrients is freely available in soil water ready to be absorbed by the roots. The chart to the right shows how different essential elements become more or less available to a plant depending on pH. You will notice towards the bottom of the chart that iron and manganese drop off quickly as a soil become more alkaline. It is not unusual to see iron and manganese deficiency in our area, but native plants and other plants that thrive here are adapted to deal with our soil conditions so they don't have this problem. Plants from the east coast, like rhododendrons and azaleas, are used to acidic soils, and they often fail in our landscapes. Testing pH is easy, and you can usually pick up a kit at your local garden center.
CEC stands for cation exchange capacity. This is a little more complicated than pH, so we'll just skim over the high points. Many of the plant nutrient ions taken up by the roots are positively charged. Certain soil particles are negatively charged, and they can attract these positive nutrient ions like a magnet and keep them from washing out of the reach of the plant's roots. Clay soils and organic materials are the best at attracting positive nutrient ions, so we say that they have a high CEC. Since we have a lot of clay in our local soils, we usually have no problems with low CEC.
Soil Biology
An impressive range of organisms lives in the soil, from single-celled bacteria to rodents. These creatures can both help and harm plants in a variety of ways. Helpful organisms can increase nutrient availability, reduce compaction, and control harmful organisms that can cause disease or injury. Right now we'll focus on how larger soil creatures and microscopic life forms benefit plants in the landscape.
Macroscopic Life
You may not usually investigate your soil for life unless you have a mole or grub problem, but the root zone of your yard is teeming with life that you can see with the naked eye. One of the biggest benefits these animals provide is aeration. The tunnels dug by ants, worms, snakes, and even troublesome moles prevent and alleviate soil compaction (we don't want moles in our lawns for other reasons, but now you can look on the bright side if you do have them). Water and air move freely through these passageways and promote root health. Our friends can also enrich the soil by leaving nutrient-rich waste and helping microorganisms with decomposition.
Microscopic Life
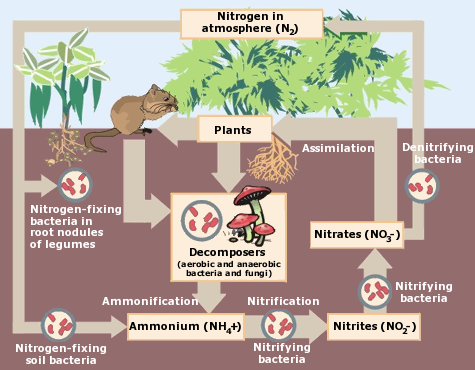
The primary way in which soil bacteria, protozoans, and their other tiny comrades benefit plants is by making essential nutrients accessible, either by working in the soil or in direct association with the plant itself. Organic matter in the soil must be broken down into smaller molecules that roots can absorb. Soil microbes are especially important in the nitrogen cycling process that converts nitrogen into forms plants can use. The image on the right gives an overview of how they help.
One fascinating example of microorganisms interacting directly with plants is called the mycorrhizal association. Mycorrhizae are fungi that live within plant roots. The fungi benefit by using some of the carbohydrates stored in the roots, and the plants benefit through increased root growth and greater root hair surface area. Plants with mycorrhizal associations root more easily, and are less susceptible to stress from lack of nutrients and water. Mycorrhizae are naturally present in healthy soil, but they can be added to poor soils to boost plant health and root development.
Our next installment will be the last in our Dirt Rocks series, and it will focus on the special challenges presented by urban and suburban soils. I will also talk about miscellaneous soil-related topics, like the difference between soil and potting mixes. Tune in for one more chapter of soil fun!
Additional Resources
Soil pH and the Availability of Nutrients (4R Nutrient Stewardship)
Fundamentals of Soil Cation Exchange Capacity (Purdue University Extension)
Soil Biology Primer (USDA Natural Resources and Conservation Service)
Hidden Partners: Mycorrhizal Fungi and Plants (New York Botanical Garden)